
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
- Diabetes Metab J. 2024;48(1):159-160. Published online January 29, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0458
- 662 View
- 93 Download
- Basic Research
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition
- Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
- Diabetes Metab J. 2023;47(6):784-795. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0261
- 1,437 View
- 149 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
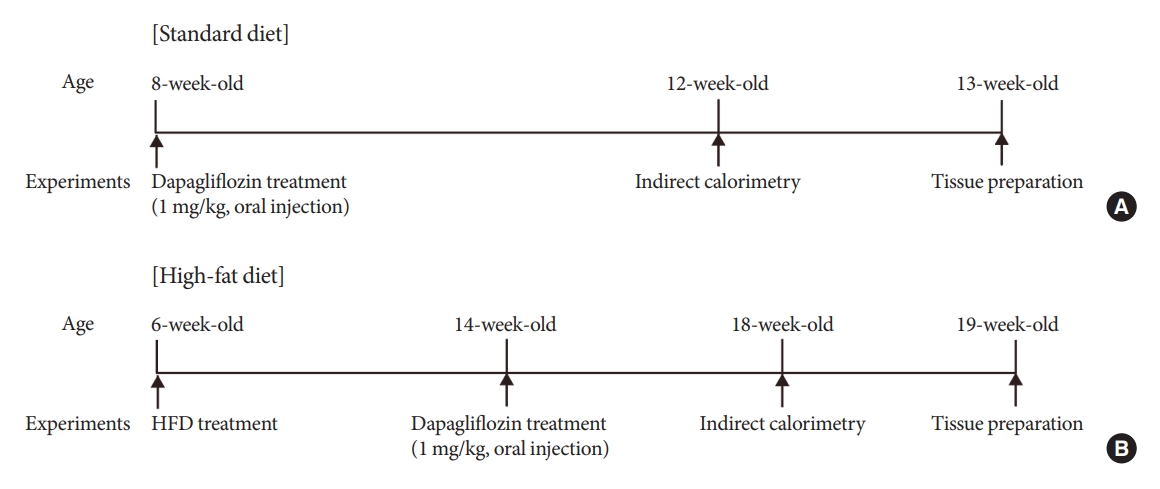
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are currently used to treat patients with diabetes. Previous studies have demonstrated that treatment with SGLT-2 inhibitors is accompanied by altered metabolic phenotypes. However, it has not been investigated whether the hypothalamic circuit participates in the development of the compensatory metabolic phenotypes triggered by the treatment with SGLT-2 inhibitors.
Methods
Mice were fed a standard diet or high-fat diet and treated with dapagliflozin, an SGLT-2 inhibitor. Food intake and energy expenditure were observed using indirect calorimetry system. The activity of hypothalamic neurons in response to dapagliflozin treatment was evaluated by immunohistochemistry with c-Fos antibody. Quantitative real-time polymerase chain reaction was performed to determine gene expression patterns in the hypothalamus of dapagliflozin-treated mice.
Results
Dapagliflozin-treated mice displayed enhanced food intake and reduced energy expenditure. Altered neuronal activities were observed in multiple hypothalamic nuclei in association with appetite regulation. Additionally, we found elevated immunosignals of agouti-related peptide neurons in the paraventricular nucleus of the hypothalamus.
Conclusion
This study suggests the functional involvement of the hypothalamus in the development of the compensatory metabolic phenotypes induced by SGLT-2 inhibitor treatment. -
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
Diabetes & Metabolism Journal.2024; 48(1): 159. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Drug/Regimen
- Efficacy and Safety of Treatment with Quadruple Oral Hypoglycemic Agents in Uncontrolled Type 2 Diabetes Mellitus: A Multi-Center, Retrospective, Observational Study
- Jun Sung Moon, Sunghwan Suh, Sang Soo Kim, Heung Yong Jin, Jeong Mi Kim, Min Hee Jang, Kyung Ae Lee, Ju Hyung Lee, Seung Min Chung, Young Sang Lyu, Jin Hwa Kim, Sang Yong Kim, Jung Eun Jang, Tae Nyun Kim, Sung Woo Kim, Eonju Jeon, Nan Hee Cho, Mi-Kyung Kim, Hye Soon Kim, Il Seong Nam-Goong, Eun Sook Kim, Jin Ook Chung, Dong-Hyeok Cho, Chang Won Lee, Young Il Kim, Dong Jin Chung, Kyu Chang Won, In Joo Kim, Tae Sun Park, Duk Kyu Kim, Hosang Shon
- Diabetes Metab J. 2021;45(5):675-683. Published online August 12, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0107
- 35,387 View
- 367 Download
- 9 Web of Science
- 5 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
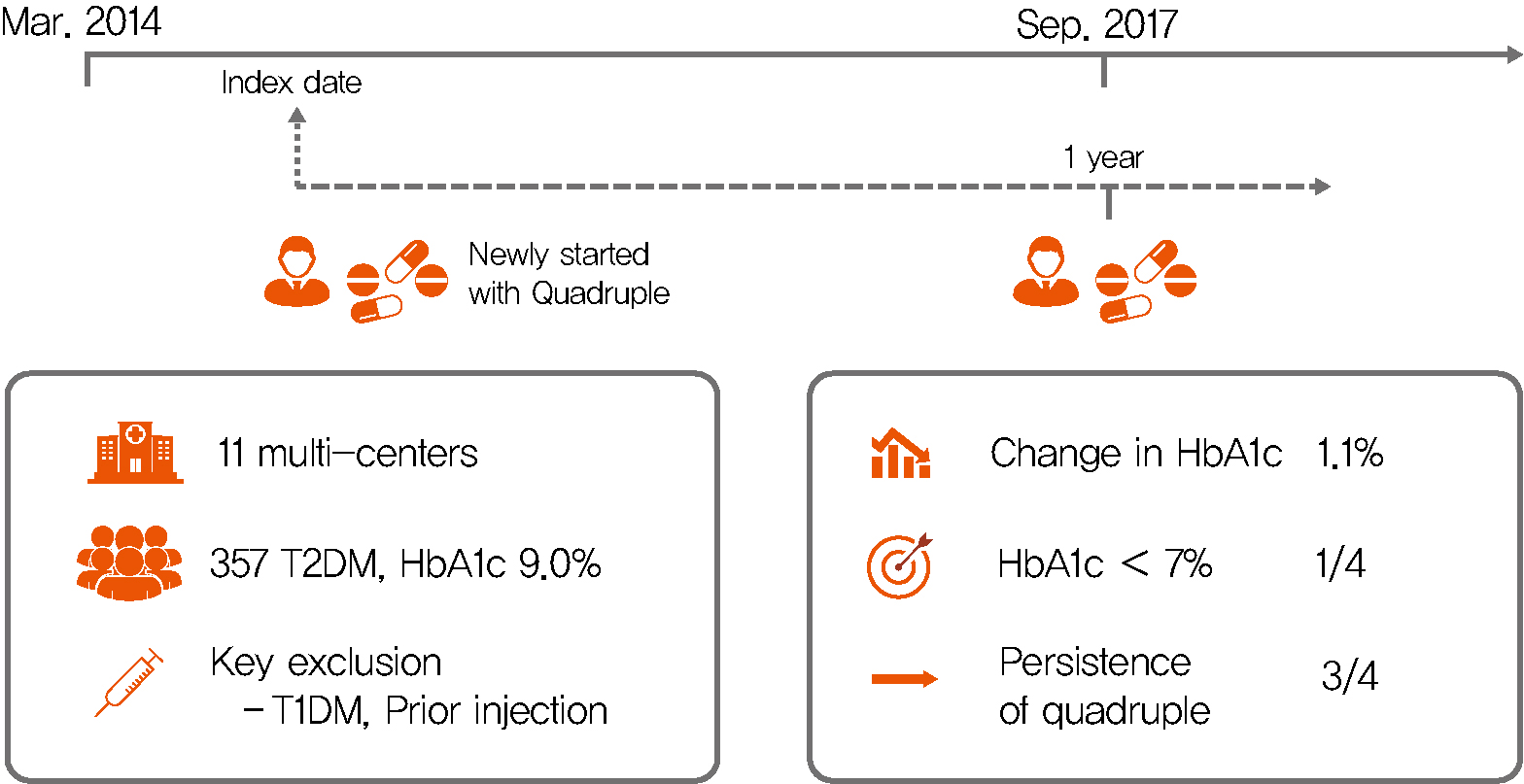
Background Only few studies have shown the efficacy and safety of glucose-control strategies using the quadruple drug combination. Therefore, the aim of the present study was to investigate the usefulness of the quadruple combination therapy with oral hypoglycemic agents (OHAs) in patients with uncontrolled type 2 diabetes mellitus (T2DM).
Methods From March 2014 to December 2018, data of patients with T2DM, who were treated with quadruple hypoglycemic medications for over 12 months in 11 hospitals in South Korea, were reviewed retrospectively. We compared glycosylated hemoglobin (HbA1c) levels before and 12 months after quadruple treatment with OHAs. The safety, maintenance rate, and therapeutic patterns after failure of the quadruple therapy were also evaluated.
Results In total, 357 patients were enrolled for quadruple OHA therapy, and the baseline HbA1c level was 9.0%±1.3% (74.9±14.1 mmol/mol). After 12 months, 270 patients (75.6%) adhered to the quadruple therapy and HbA1c was significantly reduced from 8.9%±1.2% to 7.8%±1.3% (mean change, −1.1%±1.2%;
P <0.001). The number of patients with HbA1c <7% increased significantly from 5 to 68 (P <0.005). In addition, lipid profiles and liver enzyme levels were also improved whereas no changes in body weight. There was no significant safety issue in patients treated with quadruple OHA therapy.Conclusion This study shows the therapeutic efficacy of the quadruple OHA regimen T2DM and demonstrates that it can be an option for the management of T2DM patients who cannot use insulin or reject injectable therapy.
-
Citations
Citations to this article as recorded by- Estimating Type 2 Diabetes Prevalence: A Model of Drug Consumption Data
Rita Oliveira, Matilde Monteiro-Soares, José Pedro Guerreiro, Rúben Pereira, António Teixeira-Rodrigues
Pharmacy.2024; 12(1): 18. CrossRef - Efficacy and safety of enavogliflozin versus dapagliflozin added to metformin plus gemigliptin treatment in patients with type 2 diabetes: A double-blind, randomized, comparator-active study: ENHANCE-D study
Kyung-Soo Kim, Kyung Ah Han, Tae Nyun Kim, Cheol-Young Park, Jung Hwan Park, Sang Yong Kim, Yong Hyun Kim, Kee Ho Song, Eun Seok Kang, Chul Sik Kim, Gwanpyo Koh, Jun Goo Kang, Mi Kyung Kim, Ji Min Han, Nan Hee Kim, Ji Oh Mok, Jae Hyuk Lee, Soo Lim, Sang S
Diabetes & Metabolism.2023; 49(4): 101440. CrossRef - Effectiveness and safety of teneligliptin added to patients with type 2 diabetes inadequately controlled by oral triple combination therapy: A multicentre, randomized, double‐blind, and placebo‐controlled study
Minyoung Lee, Woo‐je Lee, Jae Hyeon Kim, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2022; 24(6): 1105. CrossRef - A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
Soree Ryang, Sang Soo Kim, Ji Cheol Bae, Ji Min Han, Su Kyoung Kwon, Young Il Kim, Il Seong Nam‐Goong, Eun Sook Kim, Mi‐kyung Kim, Chang Won Lee, Soyeon Yoo, Gwanpyo Koh, Min Jeong Kwon, Jeong Hyun Park, In Joo Kim
Diabetes, Obesity and Metabolism.2022; 24(9): 1800. CrossRef - Glycaemic control with add‐on thiazolidinedione or a sodium‐glucose co‐transporter‐2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: A 24‐week, randomized controlled trial
Jaehyun Bae, Ji Hye Huh, Minyoung Lee, Yong‐Ho Lee, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2021; 23(2): 609. CrossRef
- Estimating Type 2 Diabetes Prevalence: A Model of Drug Consumption Data
- Safety and Efficacy of Modern Insulin Analogues
- Hye Jin Yoo, Keun Yong Park, Kang Seo Park, Kyu Jeung Ahn, Kyung Wan Min, Jeong Hyun Park, Sang Ah Chang, Bong Soo Cha, Dong-Jun Kim, Yong Seong Kim, Tae Keun Oh, Suk Chon, Il Seong Nam-Goong, Mi Jin Kim, Hye-Soon Kim, Young Sik Choi, You Hern Ahn, Sora Lee, Sei Hyun Baik
- Diabetes Metab J. 2013;37(3):181-189. Published online June 14, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.3.181
- 4,132 View
- 32 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background A1chieve® was a noninterventional study evaluating the clinical safety and efficacy of biphasic insulin aspart 30, insulin detemir, and insulin aspart.
Methods Korean type 2 diabetes patients who have not been treated with the study insulin or have started it within 4 weeks before enrollment were eligible for the study. The patient selection and the choice of regimen were at the discretion of the physician. The safety and efficacy information was collected from the subjects at baseline, week 12, and week 24. The number of serious adverse drug reactions (SADRs) was the primary endpoint. The changes of clinical diabetic markers at week 12 and/or at week 24 compared to baseline were the secondary endpoints.
Results Out of 4,058 exposed patients, 3,003 completed the study. During the study period, three SADRs were reported in three patients (0.1%). No major hypoglycemic episodes were observed and the rate of minor hypoglycemic episodes marginally decreased during 24 weeks (from 2.77 to 2.42 events per patient-year). The overall quality of life score improved (from 66.7±15.9 to 72.5±13.5) while the mean body weight was slightly increased (0.6±3.0 kg). The 24-week reductions in glycated hemoglobin, fasting plasma glucose and postprandial plasma glucose were 1.6%±2.2%, 2.5±4.7 mmol/L, and 4.0±6.4 mmol/L, respectively.
Conclusion The studied regimens showed improvements in glycemic control with low incidence of SADRs, including no incidence of major hypoglycemic episodes in Korean patients with type 2 diabetes.
-
Citations
Citations to this article as recorded by- Insulin therapy for adult patients with type 2 diabetes mellitus: a position statement of the Korean Diabetes Association, 2017
Byung-Wan Lee, Jin Hwa Kim, Seung-Hyun Ko, Kyu Yeon Hur, Nan-Hee Kim, Sang Youl Rhee, Hyun Jin Kim, Min Kyong Moon, Seok-O Park, Kyung Mook Choi
The Korean Journal of Internal Medicine.2017; 32(6): 967. CrossRef - Insulin Therapy for Adult Patients with Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association, 2017
Byung-Wan Lee, Jin Hwa Kim, Seung-Hyun Ko, Kyu-Yeon Hur, Nan-Hee Kim, Sang Youl Rhee, Hyun Jin Kim, Min Kyong Moon, Seok-O Park, Kyung Mook Choi
Diabetes & Metabolism Journal.2017; 41(5): 367. CrossRef - An information and communication technology-based centralized clinical trial to determine the efficacy and safety of insulin dose adjustment education based on a smartphone personal health record application: a randomized controlled trial
Gyuri Kim, Ji Cheol Bae, Byoung Kee Yi, Kyu Yeon Hur, Dong Kyung Chang, Moon-Kyu Lee, Jae Hyeon Kim, Sang-Man Jin
BMC Medical Informatics and Decision Making.2017;[Epub] CrossRef - Characteristics Predictive for a Successful Switch from Insulin Analogue Therapy to Oral Hypoglycemic Agents in Patients with Type 2 Diabetes
Gyuri Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Yonsei Medical Journal.2016; 57(6): 1395. CrossRef - Avoiding or coping with severe hypoglycemia in patients with type 2 diabetes
Jae-Seung Yun, Seung-Hyun Ko
The Korean Journal of Internal Medicine.2015; 30(1): 6. CrossRef - Clinical Characteristics of Patients Responding to Once-Daily Basal Insulin Therapy in Korean Subjects with Type 2 Diabetes
Sun Ok Song, You-Cheol Hwang, Kyu-Jeung Ahn, Bong Soo Cha, Young Duk Song, Dae Wook Lee, Byung-Wan Lee
Diabetes Therapy.2015; 6(4): 547. CrossRef - The optimal morning:evening ratio in total dose of twice‐daily biphasic insulin analogue in poorly controlled Type 2 diabetes: a 24‐week multi‐centre prospective, randomized controlled, open‐labelled clinical study
C. H. Jung, J.‐Y. Park, J. H. Cho, K.‐H. Yoon, H. K. Yang, Y.‐H. Lee, B. S. Cha, B.‐W. Lee
Diabetic Medicine.2014; 31(1): 68. CrossRef -
The glycemic efficacies of insulin analogue regimens according to baseline glycemic status in Korean patients with type 2 diabetes: sub‐analysis from the A
1
chieve
®
study
Y.‐C. Hwang, J. G. Kang, K. J. Ahn, B. S. Cha, S.‐H. Ihm, S. Lee, M. Kim, B.‐W. Lee
International Journal of Clinical Practice.2014; 68(11): 1338. CrossRef - Letter: Efficacy and Safety of Biphasic Insulin Aspart 30/70 in Type 2 Diabetes Suboptimally Controlled on Oral Antidiabetic Therapy in Korea: A Multicenter, Open-Label, Single-Arm Study (Diabetes Metab J2013;37:117-24)
Byung-Wan Lee
Diabetes & Metabolism Journal.2013; 37(3): 212. CrossRef
- Insulin therapy for adult patients with type 2 diabetes mellitus: a position statement of the Korean Diabetes Association, 2017
- Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats
- Jin Woo Lee, Il Seong Nam-Goong, Jae Geun Kim, Chang Ho Yun, Se Jin Kim, Jung Il Choi, Young IL Kim, Eun Sook Kim
- Korean Diabetes J. 2010;34(3):191-199. Published online June 30, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.3.191
- 4,535 View
- 25 Download
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Inflammation plays a role in the response to metabolic stress in type 2 diabetes. However, the effects of rosiglitazone on inflammation of skeletal muscle have not been fully examined in type 2 diabetes.
Methods We investigated the effects of the insulin-sensitizing anti-diabetic agent, rosiglitazone, on the progression of skeletal muscle inflammation in Otsuka Long-Evans Tokushima Fatty (OLETF) type 2 diabetic rats. We examined the expression of serologic markers (serum glucose, insulin and free fatty acid) and inflammatory cytokines (tumor-necrosis factor-α, interleukin [IL]-1β and IL-6) in OLETF rats from early to advanced diabetic stage (from 28 to 40 weeks of age).
Results Serum glucose and insulin concentrations were significantly decreased in rosiglitazone-treated OLETF rats compared to untreated OLETF rats. Rosiglitazone treatment significantly decreased the concentrations of serum inflammatory cytokines from 28 to 40 weeks of age. The mRNA expression of various cytokines in skeletal muscle was reduced in rosiglitazone-treated OLETF rats compared with untreated OLETF rats. Furthermore, rosiglitazone treatment resulted in the downregulation of ERK1/2 phosphorylation and NF-κB expression in the skeletal muscle of OLETF rats.
Conclusion These results suggest that rosiglitazone may improve insulin sensitivity with its anti-inflammatory effects on skeletal muscle.
-
Citations
Citations to this article as recorded by- Rosiglitazone Elicits an Adiponectin-Mediated Insulin-Sensitizing Action at the Adipose Tissue-Liver Axis in Otsuka Long-Evans Tokushima Fatty Rats
Jia Li, Yao-Ming Xue, Bo Zhu, Yong-Hua Pan, Yan Zhang, Chunxia Wang, Yuhao Li
Journal of Diabetes Research.2018; 2018: 1. CrossRef - Sirt1 and Sirt6 Mediate Beneficial Effects of Rosiglitazone on Hepatic Lipid Accumulation
Soo Jin Yang, Jung Mook Choi, Eugene Chang, Sung Woo Park, Cheol-Young Park, Aimin Xu
PLoS ONE.2014; 9(8): e105456. CrossRef - Beneficial effects of co-enzyme Q10 and rosiglitazone in fructose-induced metabolic syndrome in rats
Suzan M. Mansour, Hala F. Zaki, Ezz-El-Din S. El-Denshary
Bulletin of Faculty of Pharmacy, Cairo University.2013; 51(1): 13. CrossRef - Chromium Picolinate and Rosiglitazone Improve Biochemical Derangement in a Rat Model of Insulin Resistance: Role of TNF-a and Leptin
Suzan M. Mansour, Hala F. Zaki, Ezz-El-Din El-Denshar
Pharmacologia.2013; 4(3): 186. CrossRef - Angiotensin Receptor Blockade Increases Pancreatic Insulin Secretion and Decreases Glucose Intolerance during Glucose Supplementation in a Model of Metabolic Syndrome
Ruben Rodriguez, Jose A. Viscarra, Jacqueline N. Minas, Daisuke Nakano, Akira Nishiyama, Rudy M. Ortiz
Endocrinology.2012; 153(4): 1684. CrossRef - Rodent Models for Metabolic Syndrome Research
Sunil K. Panchal, Lindsay Brown
Journal of Biomedicine and Biotechnology.2011; 2011: 1. CrossRef - Letter: Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats (Korean Diabetes J 2010;34:191-9)
Soo Jin Yang, Cheol-Young Park
Korean Diabetes Journal.2010; 34(4): 261. CrossRef
- Rosiglitazone Elicits an Adiponectin-Mediated Insulin-Sensitizing Action at the Adipose Tissue-Liver Axis in Otsuka Long-Evans Tokushima Fatty Rats
- The Role of Hypothalamic FoxO1 on Hyperphagia in Streptozotocin-Induced Diabetic Mice.
- Il Seong Nam-Goong, Jae Geun Kim, Se Jin Kim, Seong Jae Hur, Jin Woo Lee, Eun Sook Kim, Chang Ho Yun, Byung Ju Lee, Young Il Kim
- Korean Diabetes J. 2009;33(5):375-381. Published online October 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.5.375
- 1,950 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Streptozotocin-induced diabetic animals are characterized by hyperphagia due to deficiencies of insulin and leptin. Forkhead box-containing protein of the O subfamily-1 (FoxO1) regulates energy homeostasis by regulating energy expenditure and food intake as well as mediating insulin and leptin signals in the hypothalamus. To identify the mediator of diabetic hyperphagia, we examined the effects of insulin or leptin on hypothalamic FoxO1 expression in a diabetic animal model. METHODS: Diabetes was induced in mice (C57BL/6) by intraperitoneal administration of streptozotocin (200 mg/kg). Stainless steel cannula was implanted into the lateral ventricle of the brain in each mouse. After three weeks, the mice were administered saline, insulin or leptin via intracerebroventricular (ICV) route. The medial hypothalamus was isolated to evaluate the mRNA expressions of FoxO1 and neuropeptides. RESULTS: Streptozotocin-induced diabetic mice exhibited significant elevations of blood glucose and food intake and significantly low levels of serum insulin and leptin. The levels of hypothalamic FoxO1 mRNA were significantly increased in diabetic mice. The hypothalamic expression of neuropeptide Y (NPY) mRNA was increased, but the expression of preproopiomelanocortin (POMC) mRNA was decreased in diabetic mice. ICV administration of insulin or leptin attenuated the upregulation of hypothalamic FoxO1 mRNA, and resulted in downregulation of NPY mRNA and upregulation of POMC mRNA in diabetic mice. CONCLUSION: We observed that the expression of hypothalamic FoxO1 mRNA was increased in streptozotocin-induced diabetic mice, and that it was significantly attenuated by central administration of insulin or leptin. These results suggest that hypothalamic FoxO1 is the direct mediator of diabetic hyperphagia.

 KDA
KDA
 First
First Prev
Prev





